Chemistry, 22.11.2019 20:31 alexfvdsdfgfd8151
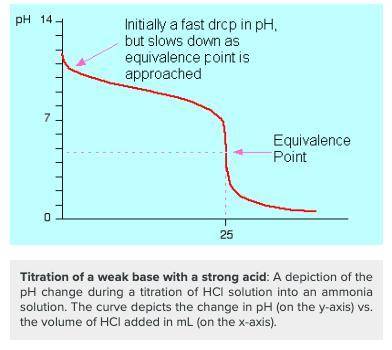
Which of the following would you identify a titration curve that involved a strong acid titrated by a weak base?
a. the ph at the equivalence point is lower than 7.
b. the ph at the equivalence point is higher than 7.
c. the titration curve begins at a higher ph and ends at a lower ph.
d. there is a rapid change in ph near the equivalence point (ph = 7).

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 00:30
Which best describes why nh4+ can form an ionic bond with ci-?
Answers: 1

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 06:30
How many moles of carbon dioxide will form if 2.5 moles of c3h8 is burned
Answers: 1
You know the right answer?
Which of the following would you identify a titration curve that involved a strong acid titrated by...
Questions

Mathematics, 12.11.2020 03:40

Mathematics, 12.11.2020 03:40



Mathematics, 12.11.2020 03:40

Mathematics, 12.11.2020 03:40


Mathematics, 12.11.2020 03:40



Biology, 12.11.2020 03:40


Mathematics, 12.11.2020 03:40



Mathematics, 12.11.2020 03:40


Mathematics, 12.11.2020 03:40