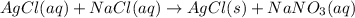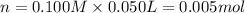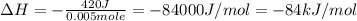Chemistry, 22.11.2019 19:31 jadabecute3739
 + nacl(aq) \rightarrow agcl(s)\ +\ nano _3(aq)) in an experiment a student mixes a 50.0 ml sample of 0.100 m agno₃ (aq) with a 50.0 ml sample of 0.100 m nacl(aq) at 20.0°c in a coffee-cup calorimeter. which of the following is the enthalpy change of the precipitation reaction represented above if the final temperature of the mixture is 21.0°c? (assume that the total mass of the mixture is 100. g and that the specific heat capacity of the mixture is 4.2 j/(g.° (a) -84 kj/mol, (b) -0.42 kj/mol (c) 0.42 kj/molu (d) 84 kj/molpx
in an experiment a student mixes a 50.0 ml sample of 0.100 m agno₃ (aq) with a 50.0 ml sample of 0.100 m nacl(aq) at 20.0°c in a coffee-cup calorimeter. which of the following is the enthalpy change of the precipitation reaction represented above if the final temperature of the mixture is 21.0°c? (assume that the total mass of the mixture is 100. g and that the specific heat capacity of the mixture is 4.2 j/(g.° (a) -84 kj/mol, (b) -0.42 kj/mol (c) 0.42 kj/molu (d) 84 kj/molpx

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 05:40
Calculate: select the worksheet tab. this tab you calculate the analyte concentration. fill in the first set of boxes ("moles h2so4" and "moles naoh") based on the coefficients in the balanced equation. (if there is no coefficient, the value is 1.) record the appropriate volumes in the "ml naoh" and "ml h2so4" boxes. record the concentration of the titrant in the m naoh box. click calculate. what is the concentration listed
Answers: 2

Chemistry, 22.06.2019 06:00
One does not belong why? ice, gold ,wood ,diamond and table salt
Answers: 1

Chemistry, 22.06.2019 07:30
In a reaction (at equilibrium) that makes more moles of gas than it consumes, what is the effect of increasing the pressure?
Answers: 1

Chemistry, 22.06.2019 20:00
How are the terms group and period used on the periodic table
Answers: 1
You know the right answer?
[tex]agno _3(aq) + nacl(aq) \rightarrow agcl(s)\ +\ nano _3(aq)[/tex]in an experiment a student mixe...
Questions


Mathematics, 10.12.2020 01:00

Arts, 10.12.2020 01:00





Mathematics, 10.12.2020 01:00

English, 10.12.2020 01:00


English, 10.12.2020 01:00

Mathematics, 10.12.2020 01:00

Geography, 10.12.2020 01:00


History, 10.12.2020 01:00





Mathematics, 10.12.2020 01:00

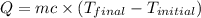
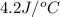
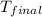 = final temperature =
= final temperature = 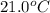
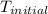 = initial temperature =
= initial temperature = 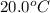
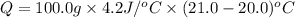
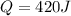
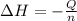
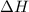 = enthalpy change = ?
= enthalpy change = ?