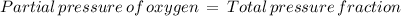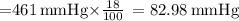Chemistry, 22.11.2019 09:31 nevaehkirk1997
The atmospheric pressure at this altitude is 461 mmhg. assuming that the atmosphere is 18% oxygen (by volume), calculate the partial pressure of o2.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3


Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2

Chemistry, 22.06.2019 20:30
Draw a line graph showing the relationship between temperature in kelvin as a function of kinetic energy.
Answers: 3
You know the right answer?
The atmospheric pressure at this altitude is 461 mmhg. assuming that the atmosphere is 18% oxygen (b...
Questions







Mathematics, 24.06.2019 23:00


English, 24.06.2019 23:00


Mathematics, 24.06.2019 23:00


Mathematics, 24.06.2019 23:00

Social Studies, 24.06.2019 23:00

Business, 24.06.2019 23:00

Social Studies, 24.06.2019 23:00


Social Studies, 24.06.2019 23:00

Social Studies, 24.06.2019 23:00