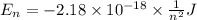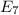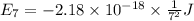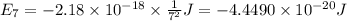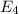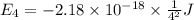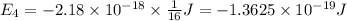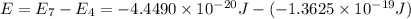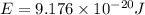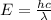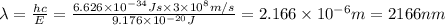Answers: 3


Another question on Chemistry

Chemistry, 23.06.2019 09:30
Need , hurry pls create a superhero out of the element iron, what are its powers and his sidekick ( an element that works well with iron). how was the superhero made and who discovered him
Answers: 3

Chemistry, 23.06.2019 16:10
Which is the best term to use when describing the energy of position? chemical kinetic potential electromagnetic
Answers: 3

Chemistry, 23.06.2019 21:50
41. we want to mark off a thermometer in both celsius and fahrenheit temperatures. on the celsius scale, the lowest temperature mark is at and the highest temperature mark is at 50 °c. what are the equivalent fahrenheit temperatures? petrucci, ralph h.. general chemistry (p. 28). pearson education. kindle edition.
Answers: 3

Chemistry, 24.06.2019 01:00
Which of dalton's ideas on atomic theory have been applied to current theory? check all that apply. a.all atoms are the same color. b.atoms can bond to and un-bond from each other. c.atoms are made up of smaller particles. d.all matter is made up of units called atoms?
Answers: 2
You know the right answer?
Calculate the wavelength, in nanometers, of the light emitted by a hydrogen atom when its electron f...
Questions

Mathematics, 19.06.2021 06:30

Mathematics, 19.06.2021 06:30

Computers and Technology, 19.06.2021 06:40

Mathematics, 19.06.2021 06:50

World Languages, 19.06.2021 06:50


Mathematics, 19.06.2021 06:50

Mathematics, 19.06.2021 06:50

English, 19.06.2021 06:50

Mathematics, 19.06.2021 06:50



Business, 19.06.2021 06:50


Mathematics, 19.06.2021 06:50



Mathematics, 19.06.2021 06:50


Mathematics, 19.06.2021 06:50