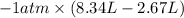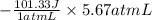Chemistry, 22.11.2019 02:31 cassandramanuel
Asample of an ideal gas in a cylinder of volume 2.67 l at 298 k and 2.81 atm expands to 8.34 l by two different pathways. path a is an isothermal, reversible expansion. path b has two steps. in the first step, the gas is cooled at constant volume to 1.00 atm. in the second step, the gas is heated and allowed to expand against a constant external pressure of 1.00 atm until the final volume is 8.34 l. calculate the work for path a and b.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 19:00
Which of the following best explains why the end of a spoon sticking out of a cup of hot water also gets hot? question 7 options: the heat from the hot water is conducted through the spoon handle the hot water heats the air surrounding the upper part of the spoon. the hot water causes a physical change in the spoon handle. the hot water causes a chemical reaction to take place in the spoon.
Answers: 2

Chemistry, 22.06.2019 06:30
If 1.8 l of water is added to 2.5l of a 7.0 m koh solution, what is the molarity of the new solution
Answers: 1

Chemistry, 22.06.2019 09:00
Ineed to find the answer of this question because i dont understand it
Answers: 1

Chemistry, 22.06.2019 13:50
How does the motion of particles in a gas change as the gas cools
Answers: 2
You know the right answer?
Asample of an ideal gas in a cylinder of volume 2.67 l at 298 k and 2.81 atm expands to 8.34 l by tw...
Questions

English, 19.07.2021 01:20



Chemistry, 19.07.2021 01:20


Advanced Placement (AP), 19.07.2021 01:20

Mathematics, 19.07.2021 01:20

Mathematics, 19.07.2021 01:20

Business, 19.07.2021 01:20





Mathematics, 19.07.2021 01:30

English, 19.07.2021 01:30


Mathematics, 19.07.2021 01:30

Mathematics, 19.07.2021 01:30


Mathematics, 19.07.2021 01:30

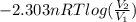
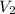 = 8.34 L,
= 8.34 L, 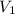 = 2.67 L
= 2.67 L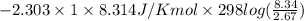
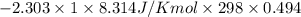
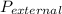 = 1.00 atm.
= 1.00 atm.