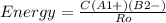Chemistry, 22.11.2019 01:31 makaylahunt
An ionic bond is formed between a cation a1 and an anion b2. how would the energy of the ionic bond [see equation (9.2)] be affected by the following changes? (a) doubling the radius of a1, (b) tripling the charge on a1, (c) doubling the charges on a1 and b2, (d) decreasing the radii of a1 and b2 to half their original values.

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 12:50
The number at the end of an isotope’s name is the number.
Answers: 1

Chemistry, 23.06.2019 03:30
Calculate the ph of a .10m nh4cl solution. the kb value for nh3 is 1.8×10^-5
Answers: 1

Chemistry, 23.06.2019 08:00
Determine the number of moles of air present in 1.35 l at 750 torr and 17.0°c. which equation should you use? n=pv/rt what is the number of moles present? ⇒ 0.056 mol a sample of n2 gas occupying 800.0 ml at 20.0°c is chilled on ice to 0.00°c. if the pressure also drops from 1.50 atm to 1.20 atm, what is the final volume of the gas? which equation should you use? v2= p1v1t2/p2t1 what is the final volume of the gas? ⇒ 932 ml these are the answers
Answers: 1
You know the right answer?
An ionic bond is formed between a cation a1 and an anion b2. how would the energy of the ionic bond...
Questions

Mathematics, 03.12.2020 19:50



Biology, 03.12.2020 19:50

Mathematics, 03.12.2020 19:50

Mathematics, 03.12.2020 19:50



Mathematics, 03.12.2020 19:50

Mathematics, 03.12.2020 19:50


Biology, 03.12.2020 19:50


Mathematics, 03.12.2020 19:50


Social Studies, 03.12.2020 19:50

Mathematics, 03.12.2020 19:50



History, 03.12.2020 19:50