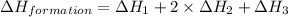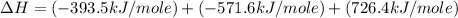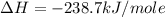Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
The nuclear fission process releases neutrons and question 27 options: alpha particles electrons energy beta particles
Answers: 1

Chemistry, 22.06.2019 17:00
The arrangement of particles is most ordered in a sample of
Answers: 1

Chemistry, 23.06.2019 06:20
Type the correct answer in each box.balance the chemical equation.__ n203 ➡️ __ n2 +__ o2
Answers: 1

Chemistry, 23.06.2019 11:30
If 4.8 moles of x and 3.4 moles of y react according to the reaction below, how many moles of the excess reactant will be left over at the end of the reaction? 3x + 2y “yields”/ x3y2. a. 1.7 mol y left over b. 1.6 mol x left over c. 0.2 mol y left over d. 0.1 mol x left over
Answers: 1
You know the right answer?
Given the following heats of combustion. ch3oh(l) + 3/2 o2(g) co2(g) + 2 h2o(l) δh°rxn = -726.4 kj c...
Questions

Mathematics, 09.12.2020 22:50



Mathematics, 09.12.2020 22:50

Mathematics, 09.12.2020 22:50

Mathematics, 09.12.2020 22:50




Mathematics, 09.12.2020 22:50

Chemistry, 09.12.2020 22:50

History, 09.12.2020 22:50


History, 09.12.2020 22:50



Geography, 09.12.2020 22:50

Mathematics, 09.12.2020 22:50

Mathematics, 09.12.2020 22:50

Computers and Technology, 09.12.2020 22:50

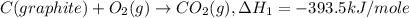 ..[1]
..[1]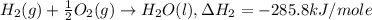 ..[2]
..[2]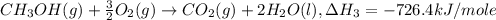 ..[3]
..[3]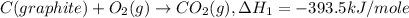 ..[1]
..[1]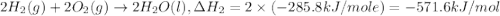 ..[2]
..[2]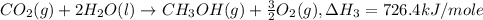 [3]
[3]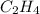 will be,
will be,