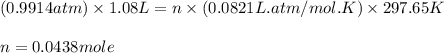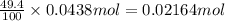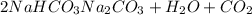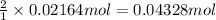You determine the volume of your plastic bag (simulated human stomach) is 1.08 l. how many grams of nahco3 (s) are required to fill this container given a 49.4% co2 recovery, assuming the other contents in the bag take up a negligible volume compared to the gas. the temperature of the room is 24.5 °c and the atmospheric pressure is 753.5 mmhg.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 02:10
3.) for each of the following compounds, draw the major organic product of reaction with hcl or naoh and circle whether the starting materials and products will be more soluble in organic solvent or water benzoic acid + hcl: benzoic acid + naoh: oh benzoic acid water/organic water organic fluorenone hс: fluorenone + naoh: fluorenone water/organic water/organic веnzocaine + hci: benzocaine + n»oh: h2n benzocaine water/organic water organic o=
Answers: 3


Chemistry, 22.06.2019 14:30
Which of the following represents the ester functional group? a. -coo- b. -cho c. -cooh d. c=o
Answers: 1

Chemistry, 22.06.2019 19:50
If a gas has an initial pressure of 101kpa and a volume of 10l, then it expands to a volume of 20l, what is the new pressure?
Answers: 2
You know the right answer?
You determine the volume of your plastic bag (simulated human stomach) is 1.08 l. how many grams of...
Questions

Mathematics, 19.06.2021 14:00


Chemistry, 19.06.2021 14:00

English, 19.06.2021 14:00



Chemistry, 19.06.2021 14:00

History, 19.06.2021 14:00

Arts, 19.06.2021 14:00

English, 19.06.2021 14:00


Mathematics, 19.06.2021 14:00

English, 19.06.2021 14:00


Social Studies, 19.06.2021 14:00

Mathematics, 19.06.2021 14:00

Social Studies, 19.06.2021 14:00

Chemistry, 19.06.2021 14:00


Biology, 19.06.2021 14:00

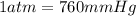 )
)