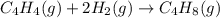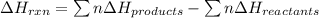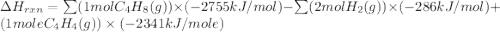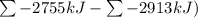Chemistry, 22.11.2019 00:31 montrellgoodman5890
Ombustion reactions involve reacting a substance with oxygen. when compounds containing carbon and hydrogen are combusted, carbon dioxide and water are the products. using the enthalpies of combustion for c4h4 (-2341 kj/mol), c4h8 (-2755 kj/mol), and h2 (-286 kj/mol), calculate δh for the reaction.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 09:00
Suppose you have designed a new thermometer called the x thermometer. on the x scale the boiling point of water is 129 ? x and the freezing point of water is 13 ? x. part a at what temperature are the readings on the fahrenheit and x thermometers the same?
Answers: 1

Chemistry, 22.06.2019 09:20
What happened to the amount of carbon dioxide in the atmosphere from 2010–2017?
Answers: 1

Chemistry, 23.06.2019 01:30
How is the solubility of a carbon dioxide gas in water increase?
Answers: 1

Chemistry, 23.06.2019 02:50
What is the typical rotational frequency frot for a molecule like n2 at room temperature (25∘c)? assume that d for this molecule is 1å=10−10m. take the total mass of an n2 molecule to be mn2=4.65×10−26kg. you will need to account for rotations around two axes (not just one) to find the correct frequency. express frot numerically in hertz, to three significant figures.
Answers: 3
You know the right answer?
Ombustion reactions involve reacting a substance with oxygen. when compounds containing carbon and h...
Questions

Biology, 07.11.2020 03:50


Mathematics, 07.11.2020 03:50

Chemistry, 07.11.2020 03:50

English, 07.11.2020 03:50


Mathematics, 07.11.2020 03:50



Mathematics, 07.11.2020 03:50

Mathematics, 07.11.2020 03:50


Mathematics, 07.11.2020 03:50

Biology, 07.11.2020 03:50

Biology, 07.11.2020 03:50

Business, 07.11.2020 03:50

Mathematics, 07.11.2020 03:50

Chemistry, 07.11.2020 03:50

Mathematics, 07.11.2020 03:50