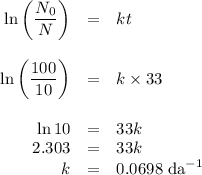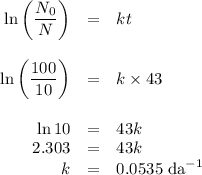Chemistry, 21.11.2019 23:31 lujaynsparkles
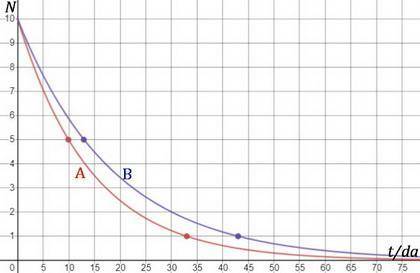
Two different radioactive isotopes decay to 10% of their respective original amounts. isotope a does this in 33 days, while isotope b does this in 43 days. what is the approximate difference in the half-lives of the isotopes? 3 days 10 days 13 days 33 days

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:30
What is the most stable monatomic ion formed from nitrogen
Answers: 2


Chemistry, 22.06.2019 14:00
What mass of natural gas (ch4) must you burn to emit 276 kj of heat?
Answers: 2

Chemistry, 22.06.2019 14:30
An object resting on a table weighs 100 n. with what force is the object pushing on the table? with what force is the table pushing on the object? explain how you got your answer.
Answers: 3
You know the right answer?
Two different radioactive isotopes decay to 10% of their respective original amounts. isotope a does...
Questions

Geography, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01



Advanced Placement (AP), 20.09.2020 07:01



History, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01

Mathematics, 20.09.2020 07:01


Mathematics, 20.09.2020 07:01

English, 20.09.2020 07:01