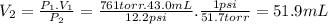Chemistry, 21.11.2019 22:31 sadiesnider9
In santa monica a dry hydrogen gas inflates a balloon to 43.0 ml at 761 torr. if the temperature remains unchanged, what if the balloons volume in denver where the pressure is 12.2 psi

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 06:00
24. a sports ball is inflated to an internal pressure of 1.85 atm at room temperature (25 °c). if the ball is then played with outside where the temperature is 7.5 °c, what will be the new pressure of the ball? assume the ball does not change in volume nor does any air leak from the ball a) 0.555 atm b) 1.74 atm c) 1.85 atm d) 1.97 atm
Answers: 2

Chemistry, 22.06.2019 10:50
How many liters of oxygen gas, at standard temperature and pressure, will react with 35.8 grams of iron metal? 4 fe (s) + 3 o₂ (g) → 2 fe₂o₃ (s)
Answers: 2

Chemistry, 22.06.2019 12:00
In the following redox reaction which is the oxidizing agent and which is the reducing agent? alcl3 + na nacl + al oxidizing agent = reducing agent =
Answers: 1

You know the right answer?
In santa monica a dry hydrogen gas inflates a balloon to 43.0 ml at 761 torr. if the temperature rem...
Questions


Spanish, 09.10.2019 22:00

Mathematics, 09.10.2019 22:00


Mathematics, 09.10.2019 22:00


Biology, 09.10.2019 22:00

Health, 09.10.2019 22:00

World Languages, 09.10.2019 22:00

English, 09.10.2019 22:00

Spanish, 09.10.2019 22:00





Business, 09.10.2019 22:00


Chemistry, 09.10.2019 22:00

Biology, 09.10.2019 22:00

Mathematics, 09.10.2019 22:00