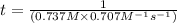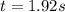Chemistry, 21.11.2019 21:31 treytonmesser
The reaction a → products was found to be second order order and have a rate constant, k, of 0.707 m-1 s-1. if the initial concentration of the reaction was 0.737 m, what is the half life for the reaction?

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:10
Which of these is the result of scientific research and not engineering? a. a new shoe design that features air cushioning for more comfort and protection b. the creation of glass with uv protection. c. a conclusion about diet commonalities among diabetics. d. the development of a smaller, more compact missile.
Answers: 1

Chemistry, 21.06.2019 22:30
Using the periodic table, complete the table to describe each atom. type in your answers.a ? b? c? d? e? f?
Answers: 1


Chemistry, 23.06.2019 04:31
How does a sample of helium at 15 degree celsius compare to a sample of helium at 215 k? a) the helium at 15 degrees celsius has a higher average kinetic energy that the sample at 215 k. b) the helium at 15 degrees celsius has lower nuclear energy that the sample at 215 k. c) the helium at 15 degrees celsius has slower- moving atoms that the sample at 215 k. d) the helium at 15 degrees celsius has smaller atoms than the sample at 215 k.
Answers: 1
You know the right answer?
The reaction a → products was found to be second order order and have a rate constant, k, of 0.707 m...
Questions

Mathematics, 16.02.2022 14:00


Chemistry, 16.02.2022 14:00

Advanced Placement (AP), 16.02.2022 14:00

SAT, 16.02.2022 14:00

English, 16.02.2022 14:00


SAT, 16.02.2022 14:00



Engineering, 16.02.2022 14:00

English, 16.02.2022 14:00



Social Studies, 16.02.2022 14:00


SAT, 16.02.2022 14:00



SAT, 16.02.2022 14:00

![\frac{1}{[A]_{t}}=kt+\frac{1}{[A]_{0}}](/tpl/images/0385/0648/16aaf.png)
![[A]_{t}](/tpl/images/0385/0648/c37dd.png) is concentration of A after "t" time and
is concentration of A after "t" time and ![[A]_{0}](/tpl/images/0385/0648/48818.png) is initial concentration of A
is initial concentration of A![[A]_{t}=\frac{[A]_{0}}{2}](/tpl/images/0385/0648/2b76d.png)
![[A]_{0}=0.737M](/tpl/images/0385/0648/c3ec8.png) and
and 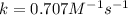
![\frac{1}{\frac{[A]_{0}}{2}}=(0.707M^{-1}s^{-1}\times t)+\frac{1}{[A]_{0}}](/tpl/images/0385/0648/7be48.png)
![\frac{1}{[A]_{0}}=0.707M^{-1}s^{-1}\times t](/tpl/images/0385/0648/fecfa.png)