

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 03:00
Compare the valence electron configuration of the nobles gas elements seen here. what statement is correct?
Answers: 2

Chemistry, 22.06.2019 07:00
Indicate whether the specified alkyl halides will form primarily substitution products, only elimination products, both substitution and elimination products, or no products when they react with sodium methoxide. 1-bromobutane 1-bromo-2-methylpropane 2-bromobutane 2-bromo-2-methylpropane
Answers: 2

Chemistry, 22.06.2019 11:40
Calculate the number of kilojoules to warm 125 g of iron from 23.5°c to 78.0°c.
Answers: 3

Chemistry, 22.06.2019 13:30
Which of the following has wavelength longer than the wavelength of viable light? a) x rays b) gamma rays c) radios waves d) ultraviolet waves
Answers: 1
You know the right answer?
Predict the sign of the entropy change of the system for each of the following reactions.
(a)...
(a)...
Questions





Mathematics, 09.04.2020 17:49

Mathematics, 09.04.2020 17:49

Mathematics, 09.04.2020 17:49







Health, 09.04.2020 17:49




Biology, 09.04.2020 17:50

Mathematics, 09.04.2020 17:50

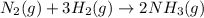
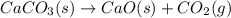
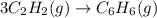
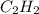 react to give 1 mole of gaseous
react to give 1 mole of gaseous 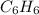 that means randomness become less that means the degree of disorderedness decreases. So, the entropy change will also decreases.
that means randomness become less that means the degree of disorderedness decreases. So, the entropy change will also decreases.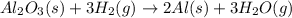
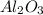 react to give 2 moles of solid aluminium that means randomness become more that means the degree of disorderedness increases. So, the entropy change will also increases.
react to give 2 moles of solid aluminium that means randomness become more that means the degree of disorderedness increases. So, the entropy change will also increases.

