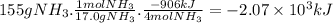Chemistry, 21.11.2019 21:31 qwertylol12345
Ammonia reacts with oxygen according to the equation 4n h 3 (g)+5 o 2 (g)→4no(g)+6 h 2 o(g),δ h rxn =−906 kj calculate the heat (in kj ) associated with the complete reaction of 155 g of n h 3 .

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 07:00
At 450 mm hg a gas has a volume of 760 l, what is its volume at standard pressure
Answers: 2


Chemistry, 22.06.2019 17:10
Some liquids can be distilled, but only at temperatures that are so high that it is impractical, or so high the compound decomposes. explain why distillation such compounds at significantly less than atmospheric pressure (some degree of vacuum) would solve this problem.
Answers: 2

Chemistry, 22.06.2019 19:20
The equation picture below shows which type of nuclear reaction u 235 + n x e 134 + sr 100 + 2n
Answers: 1
You know the right answer?
Ammonia reacts with oxygen according to the equation 4n h 3 (g)+5 o 2 (g)→4no(g)+6 h 2 o(g),δ h rxn...
Questions

Geography, 19.11.2020 18:40

Mathematics, 19.11.2020 18:40


Advanced Placement (AP), 19.11.2020 18:40


History, 19.11.2020 18:40

Health, 19.11.2020 18:40




Social Studies, 19.11.2020 18:40

History, 19.11.2020 18:40

Engineering, 19.11.2020 18:40

Chemistry, 19.11.2020 18:40

Mathematics, 19.11.2020 18:40


Mathematics, 19.11.2020 18:40


Mathematics, 19.11.2020 18:40

History, 19.11.2020 18:40