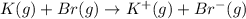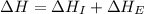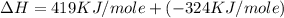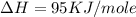Chemistry, 21.11.2019 21:31 shongmadi77
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following ionization energy (ie) and electron affinity (ea) values (hint: should one be negative for the reaction? ) ie ea k: 419 kj/mol 48 kj/mol br: 1140 kj/mol 324 kj/mol

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Consider the following reaction at equilibrium. 2co2 (g) 2co (g) + o2 (g) h° = -514 kj le châtelier's principle predicts that the equilibrium partial pressure of co (g) can be maximized by carrying out the reaction a. at high temperature and high pressure b. at high temperature and low pressure c. at low temperature and low pressure d. at low temperature and high pressure e. in the presence of solid carbon
Answers: 2

Chemistry, 22.06.2019 14:30
Connect the whole numbers on the periodic table to indicate what they represent?
Answers: 3

Chemistry, 23.06.2019 00:30
There are approximately 15 milliliters (ml) in 1 tablespoon (tbsp). what expression can be used to find the approximate number of milliliters in 3 tbsp?
Answers: 1

Chemistry, 23.06.2019 02:30
What role does weathering have in shaping earth’s surface? a) it allows sediments to fall out of a medium. b) it sediments settle on a new surface. c) it breaks down older material into sediments. d) it transports sediments to a different location. will give brainliest, answer quickly.
Answers: 2
You know the right answer?
Calculate the energy change for the reaction k(g) + br(g) → k +(g) + br – (g) given the following io...
Questions




Chemistry, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01


Mathematics, 18.10.2020 16:01


History, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01


Mathematics, 18.10.2020 16:01

Mathematics, 18.10.2020 16:01





Mathematics, 18.10.2020 16:01

History, 18.10.2020 16:01

French, 18.10.2020 16:01

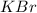 :
: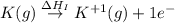
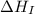 = ionization energy of potassium = 419 kJ/mol
= ionization energy of potassium = 419 kJ/mol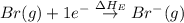
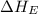 = electron affinity energy of bromine = -324 kJ/mol
= electron affinity energy of bromine = -324 kJ/mol