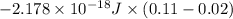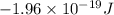Chemistry, 21.11.2019 20:31 jillianbarnes2565
An electron in the 7th energy level of the h atom drops to the 3th energy level. in other words an electron in an excited state drops to a less excited state. what is the energy (in j) of the emitted photon? the energy of an electron in the nth level

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:00
Bohr's model could only explain the spectra of which type of atoms? single atoms with one electron single atoms with more than one electron bonded atoms with one electron bonded atoms with more than one electron
Answers: 2

Chemistry, 22.06.2019 07:30
According to the vsepr theory what is the shape of a molecule that has a central atom valence three other items with no lone pairs of electrons
Answers: 1

Chemistry, 22.06.2019 09:30
1. explain hydrogen peroxide, h 2 o 2 properties and decomposition reaction. 2. describe how each of the following natural cycles plays a part in earth’s climate system. (a) the water cycle (b) the carbon cycle
Answers: 1

Chemistry, 22.06.2019 14:30
For the reaction shown, find the limiting reactant for each of the following initial amounts of reactants. 4al(s)+3o2(g)→2al2o3(s) a) 1 molal, 1 mol o2 b) 4 molal, 2.6 mol o2 c) 16 molal, 13 mol o2 d) 7.4 molal, 6.5 mol o2
Answers: 3
You know the right answer?
An electron in the 7th energy level of the h atom drops to the 3th energy level. in other words an e...
Questions




English, 01.12.2019 17:31


Health, 01.12.2019 17:31




History, 01.12.2019 17:31

History, 01.12.2019 17:31

Mathematics, 01.12.2019 17:31

History, 01.12.2019 17:31

Biology, 01.12.2019 17:31


History, 01.12.2019 17:31


Biology, 01.12.2019 17:31


Mathematics, 01.12.2019 17:31

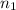 = 7,
= 7, 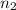 = 3
= 3![\Delta E = -2.178 \times 10^{-18} J \times (Z)^{2}[\frac{1}{n^{2}_{2}} - \frac{1}{n^{2}_{1}}]](/tpl/images/0384/9623/e92a6.png)
![-2.178 \times 10^{-18} J \times (1)^{2}[\frac{1}{(3)^{2}} - \frac{1}{(7)^{2}}]](/tpl/images/0384/9623/c0081.png)