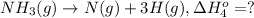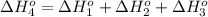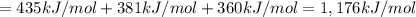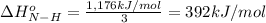Chemistry, 21.11.2019 20:31 nulledcracker12
From the following data, calculate the average bond enthalpy for the noh bond: nh3(g) ¡nh2(g) 1 h(g) ¢h° 5 435 kj/mol nh2(g) ¡nh(g) 1 h(g) ¢h° 5 381 kj/mol nh(g) ¡n(g) 1 h(g) ¢h° 5 360 kj/mol

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 23:20
Harvey kept a balloon with a volume of 348 milliliters at 25.0˚c inside a freezer for a night. when he took it out, its new volume was 322 milliliters, but its pressure was the same. if the final temperature of the balloon is the same as the freezer’s, what is the temperature of the freezer? the temperature of the freezer is kelvins.
Answers: 2

Chemistry, 22.06.2019 02:50
Using a value of ksp = 1.8 x 10-2 for the reaction pbcl2 pb+2(aq) + 2cl -(aq). if the value of ksp was determined to be only 1.2 x 10-2: too much solid has dissolved. additional precipitate is forming. the solution is unsaturated. the ions are now combining to reduce their concentrations.
Answers: 3

Chemistry, 22.06.2019 03:00
Which of these would be caused by a chemical change? a) the formation of lava. b) sedimantary rock layering over time. c) metamorphic rock forming from igneous. d) metamorphic rock eroding to form sedimentary rock.
Answers: 3

Chemistry, 22.06.2019 05:30
What type of reaction is shown below? check all that apply. 2h2o2 → 2h2o + o2 synthesis decomposition combustion
Answers: 3
You know the right answer?
From the following data, calculate the average bond enthalpy for the noh bond: nh3(g) ¡nh2(g) 1 h(g...
Questions


History, 19.09.2021 14:00


Mathematics, 19.09.2021 14:00

Mathematics, 19.09.2021 14:00

Mathematics, 19.09.2021 14:00




Mathematics, 19.09.2021 14:00

Computers and Technology, 19.09.2021 14:00


Social Studies, 19.09.2021 14:00


English, 19.09.2021 14:00


Engineering, 19.09.2021 14:00



Mathematics, 19.09.2021 14:00

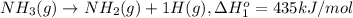 ..[1]
..[1]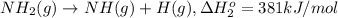 ..[2]
..[2]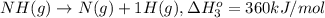 ..[3]
..[3]