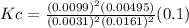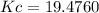Chemistry, 21.11.2019 09:31 thatonestudent2271
A100 ml reaction vessel initially contains 2.60×10^-2 moles of no and 1.30×10^-2 moles of h2. at equilibrium the concentration of no in the vessel is 0.161m. at equilibrium the vessel also contains n2, h2o, and h2. what is the value of the equilibrium constant for kc for the following reaction?
2h2 (g) + 2no(g) < —> 2h2o (g) + n2 (g)

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 00:00
Several kinds of bears are found on earth. most bears are brown or black, but one type of bear, the polar bear, is white. what process led to this difference in fur color? explain your answer.
Answers: 1


Chemistry, 22.06.2019 04:00
The continuous release of nuclear energy caused when one fission reaction triggered more nuclear reactions is a
Answers: 3

You know the right answer?
A100 ml reaction vessel initially contains 2.60×10^-2 moles of no and 1.30×10^-2 moles of h2. at equ...
Questions




Mathematics, 15.12.2020 21:30



History, 15.12.2020 21:30

English, 15.12.2020 21:30

History, 15.12.2020 21:30



English, 15.12.2020 21:30

Mathematics, 15.12.2020 21:30

Mathematics, 15.12.2020 21:30


Mathematics, 15.12.2020 21:30



Mathematics, 15.12.2020 21:30

 ml
ml moles
moles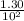 moles
moles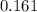 M
M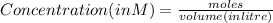
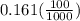
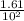 moles
moles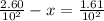
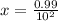
![Kc=\frac{[H2O]^2[N2]}{[H2]^2[NO]^2} (volume of vesselin litre)](/tpl/images/0384/4648/de870.png)