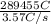Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 13:00
Metallic bonds are good conductors of electricity true or false
Answers: 2

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2


Chemistry, 23.06.2019 01:30
Concentrations expressed as a percent by mass are useful when the solute is a a. liquid b. gas c. solid
Answers: 1
You know the right answer?
How long would it take to reduce 1 mole of each of the following ions using the current indicated?...
Questions



Physics, 30.06.2019 08:30





English, 30.06.2019 08:30


Mathematics, 30.06.2019 08:30


Social Studies, 30.06.2019 08:30

History, 30.06.2019 08:30




English, 30.06.2019 08:30


Mathematics, 30.06.2019 08:30

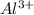 value of current is 1.234 A.
value of current is 1.234 A.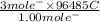
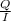
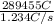
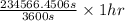
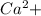 , value of current is 22.2 A.
, value of current is 22.2 A.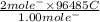
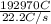
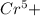 , value of current is 37.45 A.
, value of current is 37.45 A.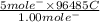
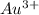 , value of current is 3.57 A.
, value of current is 3.57 A.