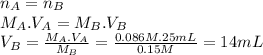Lactic acid (hc3h503) is a monoprotic acid with a k, value of 1.4 x 104 (a) what volume of 0.15 m koh would need to be added to 25 ml of 0.086 m lactic acid to reach the equivalence point? (keep 2 significant figures) ml (b) at the equivalence point, would the aqueous solution be acidic, basic, or neutral? explain why at the equivalence point, the solution will be .. at this stage, all of the lactic acid in the solution will have reacted with the koh added, producing lactate lons (c3h503") and potassium ions (*) in the solution. the potassium ions will not affect the ph, but the lactate ions will make the solution -

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Ionic compounds are made of ions, and yet the overall charge of an ionic compound is neutral. why?
Answers: 1

Chemistry, 21.06.2019 23:50
2points what is the job of a scientist? a. to answer ethical questions. b. to write laws based on his or her knowledge. c. to ask and answer scientific questions. d. to ignore facts that do not support his or her theory.
Answers: 1

Chemistry, 22.06.2019 00:50
If a reactant was removed, did the new equilibrium system shift to make more reactants or more products?
Answers: 1

Chemistry, 22.06.2019 08:00
An electron moved from shell n = 2 to shell n = 1. what most likely happened during the transition? a fraction of a photon was added. a photon of energy was absorbed. a fraction of a photon was removed. a photon of energy was released.
Answers: 1
You know the right answer?
Lactic acid (hc3h503) is a monoprotic acid with a k, value of 1.4 x 104 (a) what volume of 0.15 m ko...
Questions

Social Studies, 28.09.2019 22:50

Biology, 28.09.2019 22:50


Mathematics, 28.09.2019 22:50

Mathematics, 28.09.2019 22:50



Business, 28.09.2019 23:00


Chemistry, 28.09.2019 23:00

Spanish, 28.09.2019 23:00




Mathematics, 28.09.2019 23:00


Mathematics, 28.09.2019 23:00


Biology, 28.09.2019 23:00

Mathematics, 28.09.2019 23:00