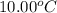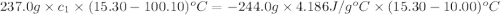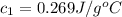A237.0 g sample of molybdenum metal is heated to 100.10 °c and then dropped into an insulated cup containing 244.0 g of water at 10.00 °c. if the final temperature of the water and metal in the cup is 15.30 °c, then what is the specific heat of molybdenum? (specific heat of water = 4.186 j/g-°c do not add the unit in the answer.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 00:00
How did planetesmals form planets? a. they broke apart into smaller chunks.b. they collided and stuck together.c. they cooled and pulled ice together.d. they began to rotate.
Answers: 1


Chemistry, 22.06.2019 07:00
Which atom or ion is the largest? a. k b. k+ c. ca d. ca2+ e. li
Answers: 1

Chemistry, 22.06.2019 10:10
For the reaction, 4 a(g) + 3 b(g) => 2 c(g), the following data were obtained at constant temperature. experiment initial[a],mol/l initial [b],mol/l initial rate,m/min 1 0.200 0.150 5.00 2 0.400 0.150 10.0 3 0.200 0.300 10.0 4 0.400 0.300 20.0 which of the following is the correct rate law for the reaction? 1. rate = k[a]2[b]2 2. rate = k[a][b] 3. rate = k[a]2[b] 4. rate = k[a][b]2
Answers: 3
You know the right answer?
A237.0 g sample of molybdenum metal is heated to 100.10 °c and then dropped into an insulated cup co...
Questions

History, 09.01.2020 08:31



Mathematics, 09.01.2020 08:31

Chemistry, 09.01.2020 08:31


History, 09.01.2020 09:31


Chemistry, 09.01.2020 09:31





History, 09.01.2020 09:31

Mathematics, 09.01.2020 09:31


Mathematics, 09.01.2020 09:31

Mathematics, 09.01.2020 09:31

Social Studies, 09.01.2020 09:31

History, 09.01.2020 09:31

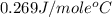
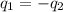
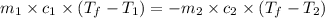
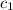 = specific heat of molybdenum metal = ?
= specific heat of molybdenum metal = ?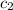 = specific heat of water =
= specific heat of water = 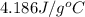
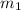 = mass of molybdenum metal = 237.0 g
= mass of molybdenum metal = 237.0 g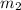 = mass of water = 244.0 g
= mass of water = 244.0 g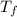 = final temperature of water and metal =
= final temperature of water and metal = 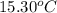
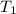 = initial temperature of molybdenum metal =
= initial temperature of molybdenum metal = 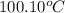
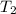 = initial temperature of water =
= initial temperature of water =