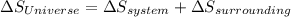Chemistry, 21.11.2019 06:31 mraymundo025p0gpw9
Identify the following statements as true or false.1) all spontaneous reactions occur quickly.2) the energy of the universe is constant; the entropy of the universe increases.3) if a process increases the randomness of the particles of a system, the entropy of the system increases.4) all systems become more disordered spontaneously.5) all spontaneous processes release heat.6) both δ ssys and δ ssurr must equal zero at equilibrium.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 21:10
When the volume and number of particles of a gas are constant which of the following is also constant
Answers: 3


Chemistry, 23.06.2019 05:30
For the reaction i2(g)+br2(g)←−→2ibr(g), kc=280 at 150 ∘c. suppose that 0.450 mol ibr in a 2.00-l flask is allowed to reach equilibrium at 150 ∘c. what is the equilibrium concentration of 2ibr, i2, br2
Answers: 1

Chemistry, 23.06.2019 12:30
An atom holds 7 electrons. use orbital notation to model the probable location of its electrons. an atom hold 22 electrons. use orbital notation to model the probable location of its electrons. an atom holds 17 electrons. use orbital notation to model the probable location of its electrons.
Answers: 1
You know the right answer?
Identify the following statements as true or false.1) all spontaneous reactions occur quickly.2) the...
Questions

Mathematics, 07.12.2020 23:20


Physics, 07.12.2020 23:20

History, 07.12.2020 23:20

Health, 07.12.2020 23:20

Biology, 07.12.2020 23:20

Mathematics, 07.12.2020 23:20

Mathematics, 07.12.2020 23:20



Business, 07.12.2020 23:20

Biology, 07.12.2020 23:20


Mathematics, 07.12.2020 23:20

Engineering, 07.12.2020 23:20

Mathematics, 07.12.2020 23:20

Mathematics, 07.12.2020 23:20

Mathematics, 07.12.2020 23:20

History, 07.12.2020 23:20

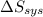 and
and 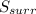 must equal zero at equilibrium.
must equal zero at equilibrium.