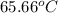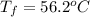Chemistry, 21.11.2019 05:31 tabocampos1414
A13.60-g block of solid aluminum at 13.91 °c is immersed in a 23.27-g pool of liquid ethylene glycol with a temperature of 65.66 °c. when thermal equilibrium is reached, what is the temperature of the aluminum and ethylene glycol? specific heat capacities: lead = 0.159 j/g °c; ethylene glycol = 2.36 j/g ° °c

Answers: 3


Another question on Chemistry


Chemistry, 22.06.2019 06:00
Which of the following did jj thompson discover about atoms? a)an atom has an internal structure. b) atoms are tiny indivisible particles. c)electrons orbit the nucleus of an atom. d) the nucleus of an atom contains protons and neutrons.
Answers: 2

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 14:30
How does a noncompetitive inhibitor reduce an enzyme’s activity?
Answers: 1
You know the right answer?
A13.60-g block of solid aluminum at 13.91 °c is immersed in a 23.27-g pool of liquid ethylene glycol...
Questions

English, 03.08.2019 02:00

Mathematics, 03.08.2019 02:00

Health, 03.08.2019 02:00



History, 03.08.2019 02:00

Mathematics, 03.08.2019 02:00

English, 03.08.2019 02:00

Biology, 03.08.2019 02:00

Mathematics, 03.08.2019 02:00


Computers and Technology, 03.08.2019 02:00

History, 03.08.2019 02:00




History, 03.08.2019 02:00



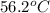
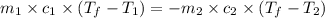
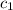 = specific heat of aluminum =
= specific heat of aluminum = 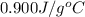
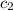 = specific heat of ethylene glycol =
= specific heat of ethylene glycol = 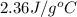
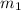 = mass of aluminum = 13.60 g
= mass of aluminum = 13.60 g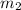 = mass of ethylene glycol = 23.27 g
= mass of ethylene glycol = 23.27 g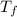 = final temperature of aluminum and ethylene glycol = ?
= final temperature of aluminum and ethylene glycol = ?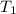 = initial temperature of aluminium =
= initial temperature of aluminium = 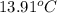
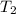 = initial temperature of ethylene glycol =
= initial temperature of ethylene glycol =