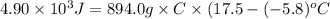Chemistry, 21.11.2019 04:31 crosales102
Achemist carefully measures the amount of heat needed to raise the temperature of a sample of a pure substance from to . the experiment shows that of heat are needed. what can the chemist report for the specific heat capacity of the substance? be sure your answer has the correct number of significant digits.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 08:50
If two atoms are bonded to a central atom with no lone pairs,how will they be arranged
Answers: 3


Chemistry, 22.06.2019 19:30
Chlorine and water react to form hydrogen chloride and oxygen, like this: 2cl2 (g) + 2h2o (g) → 4hcl (g) + o2 (g) also, a chemist finds that at a certain temperature the equilibrium mixture of chlorine, water, hydrogen chloride, and oxygen has the following composition: compound concentration at equilibrium cl2 0.55m h2o 0.57m hcl 0.53m o2 0.34m calculate the value of the equilibrium constant kc for this reaction. round your answer to 2 significant digits.
Answers: 2

Chemistry, 22.06.2019 20:30
Select all the correct answers.which compounds have the empirical formula ch20? (multiple answers)a.c2h4o2b.c3h603c.ch2o2d.c5h1005e.c6h1206
Answers: 2
You know the right answer?
Achemist carefully measures the amount of heat needed to raise the temperature of a sample of a pure...
Questions

Geography, 04.11.2019 11:31

Mathematics, 04.11.2019 11:31





Mathematics, 04.11.2019 11:31

Mathematics, 04.11.2019 11:31




Mathematics, 04.11.2019 11:31

Mathematics, 04.11.2019 11:31

Mathematics, 04.11.2019 11:31

Chemistry, 04.11.2019 11:31


Mathematics, 04.11.2019 12:31



 to
to 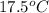 and heat needed is 4.90 kJ (or 4900 J, as 1 kJ = 1000 J).
and heat needed is 4.90 kJ (or 4900 J, as 1 kJ = 1000 J).