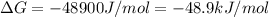Chemistry, 21.11.2019 04:31 alexreddin3127
Acritical reaction in the production of energy to do work or drive chemical reactions in biological systems is the hydrolysis of adenosine triphosphate, atp, to adenosine diphosphate, adp, as described by
atp (aq)+ h20 (l) -> adp (aq) + hpo4 (negative two overall charge) (aq).
for which ? g�rxn = �30.5 kj/mol at 37.0 �c and ph 7.0. calculate the value of ? grxn in a biological cell in which [atp] = 5.0 mm, [adp] = 0.80 mm, and [hpo42�] = 5.0 mm.
a. what is the delta g rxn in kj/mol?
b. is the hydrolysis of atp spontaneous under these conditions?

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 19:30
If 16.00g of hydrogen gas reacts with 126.73g of oxygen, how many grams of water are yielded? (both reactants are completely consumed in the reaction.)
Answers: 2

Chemistry, 22.06.2019 19:30
Describe the forces both attractive and repulsive that occur as two atoms move closer together.
Answers: 1

Chemistry, 23.06.2019 06:40
15. what volume of cci, (d = 1.6 g/cc) contain6.02 x 1025 cci, molecules (ci = 35.5)(1) 10.5 l(2) 250 ml(3) 9.625 l(4) 1.712 lplz answer with step by step explanation
Answers: 1

Chemistry, 23.06.2019 08:00
If the solubility of a gas in water is 1.22 g/l at 2.75 atm, what is its solubility (in g/l) at 1.0 atm?
Answers: 1
You know the right answer?
Acritical reaction in the production of energy to do work or drive chemical reactions in biological...
Questions


Chemistry, 12.05.2021 17:50

Mathematics, 12.05.2021 17:50

History, 12.05.2021 17:50

Mathematics, 12.05.2021 17:50



World Languages, 12.05.2021 17:50

Mathematics, 12.05.2021 17:50

Mathematics, 12.05.2021 17:50

Mathematics, 12.05.2021 17:50









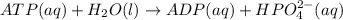
![[HPO_4^{2-}] = 5.0 mM=0.005 M](/tpl/images/0384/0544/f1ef4.png)
![Q=\frac{[ADP][HPO_4^{2-}]}{[ATP]}=\frac{0.0008 M\times 0.005 M}{0.005 M}=0.0008](/tpl/images/0384/0544/0fdb9.png)
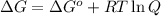
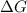 = Gibbs free energy at given conditions
= Gibbs free energy at given conditions = Gibbs free energy at equilibrium=-30.5 kJ/mol
= Gibbs free energy at equilibrium=-30.5 kJ/mol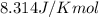
![37^oC=[273.15+37]K=310.15 K](/tpl/images/0384/0544/5ce7f.png)
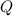 = reaction quotient at 37°C = 0.0008
= reaction quotient at 37°C = 0.0008![\Delta G=-30500 J/mol+(8.314J/Kmol)\times 310.15 K\times \ln [0.0008]](/tpl/images/0384/0544/d6332.png)