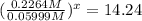At 593k a particular decomposition’s rate constant had a value of 5.21×10−4 and at 673k the same reaction’s rate constant was 7.42×10−3. it was noticed that when the reactant’s initial concentration was 0.2264 m (with a 593k reaction temperature), the initial reaction rate was identical to the initial rate when the decomposition was run at 673k with an initial reactant concentration of 0.05999 m. recall that rate laws have the form rate = k [a]x and, showing work, determine the order of the decomposition reaction.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 19:20
What is the strongest intermolecular force between an nacl unit and an h2o molecule together in a solution? covalent bonding dipole-dipole force hydrogen bonding ion-dipole force
Answers: 1

Chemistry, 22.06.2019 17:30
What will most likely happen in the absence of a cell membrane? a) photosynthesis will not take place. b) the cell will not store food, water, nutrients, and waste. c) energy will not be released during cellular respiration. d) substances will pass in and out of the cell in an uncontrolled manner.
Answers: 1

Chemistry, 22.06.2019 21:50
Liquid from a brewery fermentation contains 10% ethanol and 90% water. part of the fermentation product (50,000 kg/h) is pumped to a distillation column on the factory site. under current operating conditions, a distillate of 45% ethanol and 55% water is produced from the top of the column at a rate of one-tenth that of the feed. what is the composition of the waste "bottoms" from the still?
Answers: 2

Chemistry, 22.06.2019 22:30
You just calculated that the heat of fusion for chloromethane is 6400 j/mol. the heat of fusion for hydrogen is 120 j/mol.? which of the following account for this difference? more than one correcta. chloromethane can absorb more energy at the same temperature. b. hydrogen has stronger intermolecular forces than chloromethane. c. hydrogen molecules can pack more closely than chloromethane molecules. d. chloromethane experiences dipole-dipole interactions. e. chloromethane has a higher molar mass than hydrogen.
Answers: 3
You know the right answer?
At 593k a particular decomposition’s rate constant had a value of 5.21×10−4 and at 673k the same rea...
Questions

Mathematics, 28.09.2020 05:01

English, 28.09.2020 05:01


Mathematics, 28.09.2020 05:01

Chemistry, 28.09.2020 05:01




Mathematics, 28.09.2020 05:01

Chemistry, 28.09.2020 05:01

Mathematics, 28.09.2020 05:01


Mathematics, 28.09.2020 05:01


Mathematics, 28.09.2020 05:01


Mathematics, 28.09.2020 05:01


Mathematics, 28.09.2020 05:01

Mathematics, 28.09.2020 05:01

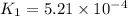
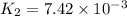
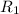
![R_1=K_1\times [A]^x](/tpl/images/0384/0720/5a42c.png)
![R_1=5.21\times 10^{-4}\times [A]^x](/tpl/images/0384/0720/5f894.png) ...[1]
...[1]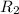
![R_2=K_2\times [A']^x](/tpl/images/0384/0720/6a78b.png)
![R_2=7.42\times 10^{-3}\times [A']^x](/tpl/images/0384/0720/658af.png) ...[2]
...[2]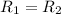 (given)
(given)![5.21\times 10^{-4}\times [A]^x=7.42\times 10^{-3}\times [A']^x](/tpl/images/0384/0720/ebaac.png)
![(\frac{[A]}{[A']})^x=\frac{7.42\times 10^{-3}}{5.21\times 10^{-4}}](/tpl/images/0384/0720/a9fcc.png)