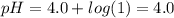Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 14:20
Which statement explains why the bonds between non metals tend to be covalent? the bonds are found to be nondirectional they have large differences in electronegativity they have small differences in electronegativity they have ions that produce an electrostatic pull
Answers: 1

Chemistry, 22.06.2019 15:30
Using the first volume and temperature reading on the table as v1 and t1, solve for the unknown values in the table below. remember to use the rules of significant figures when entering your numeric response.
Answers: 2

Chemistry, 22.06.2019 16:50
Answer asap need by wednesday morning calculate the ph of 0.16m ch3cooh which has ka = 1.74 x 10-5 mol dm-3 best answer will be brainliest
Answers: 3
You know the right answer?
Aph 4 buffer solution is prepared by dissolving one mole of a weak acid ha (pka = 4.0) and one mole...
Questions

Mathematics, 26.01.2021 04:30



English, 26.01.2021 04:30


Mathematics, 26.01.2021 04:30

English, 26.01.2021 04:30


History, 26.01.2021 04:30


Mathematics, 26.01.2021 04:30

English, 26.01.2021 04:30





Mathematics, 26.01.2021 04:30

English, 26.01.2021 04:30

Spanish, 26.01.2021 04:30

Mathematics, 26.01.2021 04:30

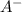 )-
)-![pH=pK_{a}(HA)+log\frac{[A^{-}]}{[HA]}](/tpl/images/0383/9401/34f32.png)
![[A^{-}]](/tpl/images/0383/9401/fe74d.png) and [HA] represents concentration (in molarity) of
and [HA] represents concentration (in molarity) of 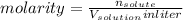
![\frac{[A^{-}]}{[HA]}=\frac{\frac{n_{A^{-}}}{V_{solution}in liter}}{\frac{n_{HA}}{V_{solution}in liter}}=\frac{\frac{1 mol}{10 L}}{\frac{1mol}{10L}}=1](/tpl/images/0383/9401/ac2ca.png)