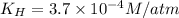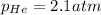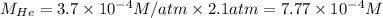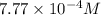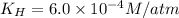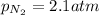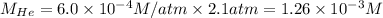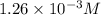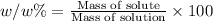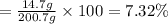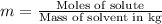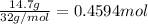Chemistry, 21.11.2019 02:31 josie17340
The henry's law constant for helium gas in water at 30 ∘c is 3.7×10−4m/atm; the constant for n2 at 30 ∘c is 6.0×10−4m/atm. a. if helium gas is present at 2.1 atm pressure, calculate the solubility of this gas. b. if n2 is present at 2.1 atm pressure, calculate the solubility of this gas.2. a solution is made containing 14.7 g of ch3oh in 186 g h2o. a. calculate the mass percent of ch3oh. b. calculate the molality of ch3oh.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 02:00
The alkali metals (group 1) consist of lithium (3), sodium (11), potassium (19), rubidium (37), cesium (55), and francium (87). they are soft, metallic solids with low densities and low melting points. based on the data shown in figure 1, how many valence electrons do alkali metals share?
Answers: 3

Chemistry, 22.06.2019 13:00
In what environment would mineral formation caused by high pressures and high temperatures most likely occur?
Answers: 3

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3

Chemistry, 22.06.2019 14:50
Given the following information: mass of proton = 1.00728 amu mass of neutron = 1.00866 amu mass of electron = 5.486 × 10^-4 amu speed of light = 2.9979 × 10^8 m/s calculate the nuclear binding energy (absolute value) of 3li^6. which has an atomic mass of 6.015126 amu. j/mol.
Answers: 2
You know the right answer?
The henry's law constant for helium gas in water at 30 ∘c is 3.7×10−4m/atm; the constant for n2 at...
Questions








Business, 04.07.2021 21:30



Mathematics, 04.07.2021 21:30

Mathematics, 04.07.2021 21:30




History, 04.07.2021 21:30


Mathematics, 04.07.2021 21:30


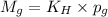
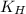 = Henry's constant
= Henry's constant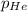 = partial pressure of gas
= partial pressure of gas