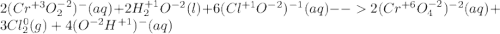Consider the following balanced redox reaction (do not include state of matter in your answers): 2cro2−(aq) + 2h2o(l) + 6clo−(aq) →2cro42−(aq) + 3cl2(g) + 4oh−(aq)(a) which species is being oxidized? (b) which species is being reduced? (c) which species is the oxidizing agent? (d) which species is the reducing agent? (e) from which species to which does electron transfer occur?

Answers: 1


Another question on Chemistry

Chemistry, 21.06.2019 14:00
Which of the following statements is true? question 4 options: nuclear decay rates vary with the conditions of the reaction, but chemical reaction rates do not. chemical reaction rates vary with the conditions of the reaction, but nuclear decay rates do not. neither chemical reaction rates nor nuclear decay rates vary with the conditions of the reaction. both chemical reaction rates and nuclear decay rates vary with the conditions of the reaction.
Answers: 1

Chemistry, 21.06.2019 16:30
What is the force of attraction between the particles in a salt crystal
Answers: 2

Chemistry, 22.06.2019 08:00
Which of the following observations indicates that there is a small, dense, positively charged part in the center of an atom? some uncharged particles are scattered by a gold foil. all uncharged particles are attracted towards a gold foil. all positively charged particles pass straight through a gold foil. some positively charged particles bounce back from a gold foil.
Answers: 2

Chemistry, 22.06.2019 08:30
In a chemical reaction at equilibrium, the rate of the forward reaction the rate of the reverse reaction. if the rate of the forward reaction more products are formed.
Answers: 1
You know the right answer?
Consider the following balanced redox reaction (do not include state of matter in your answers): 2cr...
Questions

Mathematics, 11.07.2019 08:10


Mathematics, 11.07.2019 08:10










Health, 11.07.2019 08:10


Health, 11.07.2019 08:10

Mathematics, 11.07.2019 08:10


Health, 11.07.2019 08:10

Health, 11.07.2019 08:10

Health, 11.07.2019 08:10