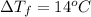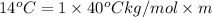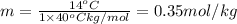Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:40
C3h8o3 - glycerol major species present when dissolved in water
Answers: 2

Chemistry, 22.06.2019 17:10
Calculate the estimated density of each ball. use the formula d = m/v where d is the density, m is the mass, and v is the volume. record your calculations in table a of your student guide. given that the density of water is 1.0 g/cm3, make a prediction about whether each ball will float in water. record your prediction in table a. what is the estimated density of the table tennis ball? record your answer to the nearest hundredth
Answers: 2


Chemistry, 23.06.2019 06:30
Consider the heating curve of h2o and line segments a, b, and c. several changes are taking place at a, b, and c. all but one would be an appropriate description as e move through segments a, b and then c.
Answers: 3
You know the right answer?
Calculate the molality of isoborneol in the product if, a) the melting point of pure camphor is 179°...
Questions

Mathematics, 01.03.2021 18:20

Mathematics, 01.03.2021 18:20




Mathematics, 01.03.2021 18:20

Mathematics, 01.03.2021 18:20


Mathematics, 01.03.2021 18:20






Mathematics, 01.03.2021 18:20





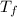 = 165°C
= 165°C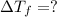
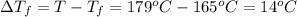
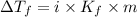
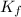 = The freezing point depression constant
= The freezing point depression constant