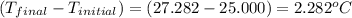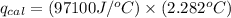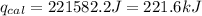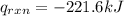Chemistry, 21.11.2019 01:31 Deadpool9609
4.41 g of propane gas (c3h8) is injected into a bomb calorimeter and ignited with excess oxygen, according to the reaction below. the calorimeter (including the water) has a heat capacity of 97.1 kj/°c. c3h8(g) + 5 o2(g) 3 co2(g) + 4 h2o() (a) if the temperature rose from 25.000°c to 27.282°c, what is the heat of the reaction, qrxn?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 03:00
How does a hydroelectric power plant converts energy into energy.
Answers: 1

Chemistry, 22.06.2019 13:00
Asubstance is a good conductor of electricity which of the following best explains a probable position of the substance in a periodic table
Answers: 3


Chemistry, 22.06.2019 19:00
Mercury metal is poured into a graduated cylinder that holds exactly 22.5 ml the mercury used to fill the cylinder mass in 306.0 g from this information calculate the density of mercury
Answers: 2
You know the right answer?
4.41 g of propane gas (c3h8) is injected into a bomb calorimeter and ignited with excess oxygen, acc...
Questions

Mathematics, 24.09.2020 21:01

Mathematics, 24.09.2020 21:01

Mathematics, 24.09.2020 21:01





Computers and Technology, 24.09.2020 21:01

Mathematics, 24.09.2020 21:01


Mathematics, 24.09.2020 21:01

Biology, 24.09.2020 21:01

Mathematics, 24.09.2020 21:01



Mathematics, 24.09.2020 21:01

Spanish, 24.09.2020 21:01



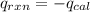
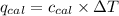
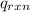 = heat released by the reaction = ?
= heat released by the reaction = ?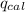 = heat absorbed by the calorimeter
= heat absorbed by the calorimeter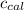 = specific heat of calorimeter =
= specific heat of calorimeter = 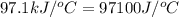
 = change in temperature =
= change in temperature =