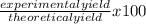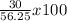Chemistry, 20.11.2019 23:31 jacobs5919
If 39.0 g of c6h6 reacts with excess chlorine and produces 30.0 g of c6h5cl in the reaction c6h6 + cl2 → c6h5cl + hcl , what is the percent yield of c6h5cl? 1. 50.0% 2. 53.4% 3. 69.4% 4. 13.2% 5. 76.9%

Answers: 2


Another question on Chemistry


Chemistry, 22.06.2019 08:00
Identify a strong intermolecular force of attraction between an alcohol
Answers: 1

Chemistry, 22.06.2019 20:00
Listenbase your answer to the question on the information below.nuclear radiation is harmful to living cells, particularly to fast-growing cells, such as cancer cells and blood cells. an external beam of the radiation emitted from a radioisotope can be directed on a small area of a person to destroy cancer cells within the body.cobalt-60 is an artificially produced radioisotope that emits gamma rays and beta particles. one hospital keeps a 100.0-gram sample of cobalt-60 in an appropriate, secure storage container for future cancer treatment.which choice represents the correct product for the beta decay of the co-60? fe-60ni-60fe-61ni-61
Answers: 2

Chemistry, 22.06.2019 20:30
Consider the following unbalanced equation for the combustion of hexane: αc6h14(g)+βo2(g)→γco2(g)+δh2o(g) part a balance the equation. give your answer as an ordered set of numbers α, β, γ, use the least possible integers for the coefficients. α α , β, γ, δ = nothing request answer part b determine how many moles of o2 are required to react completely with 5.6 moles c6h14. express your answer using two significant figures. n n = nothing mol request answer provide feedback
Answers: 2
You know the right answer?
If 39.0 g of c6h6 reacts with excess chlorine and produces 30.0 g of c6h5cl in the reaction c6h6 + c...
Questions

Mathematics, 03.05.2020 14:09

Mathematics, 03.05.2020 14:09



History, 03.05.2020 14:09

Mathematics, 03.05.2020 14:09

Mathematics, 03.05.2020 14:09


Mathematics, 03.05.2020 14:09



Mathematics, 03.05.2020 14:09

Mathematics, 03.05.2020 14:09

Mathematics, 03.05.2020 14:09


Mathematics, 03.05.2020 14:09



Mathematics, 03.05.2020 14:09

Mathematics, 03.05.2020 14:09