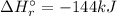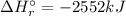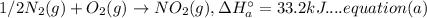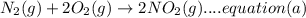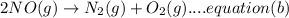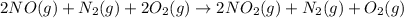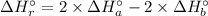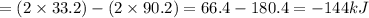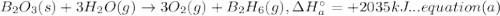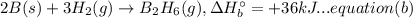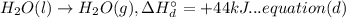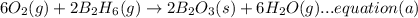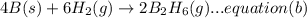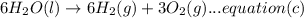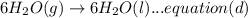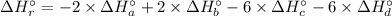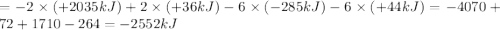Chemistry, 20.11.2019 23:31 headshotplayzcod
A. calculate the enthalpy of the reaction 2no(g)+o2(g)→2no2(g) given the following reactions and enthalpies of formation: 12n2(g)+o2(g)→no2(g), δh∘a=33.2 kj 12n2(g)+12o2(g)→no(g), δh∘b=90.2 kj express your answer with the appropriate units. b. calculate the enthalpy of the reaction4b(s)+3o2(g)→2b2o3(s)given the following pertinent information: b2o3(s)+3h2o(g)→3o2(g)+b2h6(g), δh∘a=+2035 kj2b(s)+3h2(g)→b2h6(g), δh∘b=+36 kjh2(g)+12o2(g)→h2o(l), δh∘c=−285 kjh2o(l)→h2o(g), δh∘d=+44 kjexpress your answer with the appropriate units.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 20:30
This chart represents the melting point of several substance. what besy explains the high melting point of the salt?
Answers: 2

Chemistry, 22.06.2019 07:00
This image is an example of a(n) a) atom. b) compound. c) mixture. d) molecule.
Answers: 1


Chemistry, 23.06.2019 07:50
Asolution is produced in which water is the solvent and there are four solutes. which of the solutes can dissolve better if the solution is heated?
Answers: 1
You know the right answer?
A. calculate the enthalpy of the reaction 2no(g)+o2(g)→2no2(g) given the following reactions and ent...
Questions

Chemistry, 21.05.2020 12:59







Biology, 21.05.2020 12:59




World Languages, 21.05.2020 12:59


English, 21.05.2020 12:59

Chemistry, 21.05.2020 12:59

Mathematics, 21.05.2020 12:59


Biology, 21.05.2020 12:59