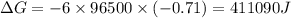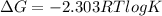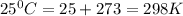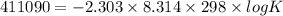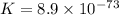Chemistry, 20.11.2019 20:31 pennyluvsu13
Use the tabulated half-cell potentials to calculate the equilibrium constant (k) for the following balanced redox reaction at 25°c. 2 al(s) + 3 mg2+(aq) → 2 al3+(aq) + 3 mg(s) a) 1.1 × 1072 b) 8.9 × 10-73 c) 1.1 × 10-72 d) 1.0 × 1024 e) 4.6 × 1031

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 22:30
Agas at 155 kpa and standard temperature has an initial volume of 1.00 l. the pressure of the gas rises to 500 kpa as the temperature also rises to 135°c. what is the new volume? 2.16 l 0.463 l 0.207 l 4.82 l
Answers: 3

Chemistry, 22.06.2019 08:00
If 90.0 grams of ethane reacted with excess chlorine,how many grams of dicarbon hexachloride would form
Answers: 1


Chemistry, 22.06.2019 09:30
Apump contains 0.5 l of air at 203 kpa.you draw back on the piston of the pump, expanding the volume until the pressure reads 50.8 kpa. what is the new volume of the air pump
Answers: 2
You know the right answer?
Use the tabulated half-cell potentials to calculate the equilibrium constant (k) for the following b...
Questions

English, 23.06.2019 22:00





Mathematics, 23.06.2019 22:00

Mathematics, 23.06.2019 22:00


Mathematics, 23.06.2019 22:00

Mathematics, 23.06.2019 22:00

English, 23.06.2019 22:00



Mathematics, 23.06.2019 22:00

Mathematics, 23.06.2019 22:00




History, 23.06.2019 22:00

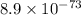
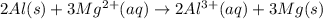
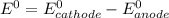
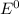 are standard reduction potentials.
are standard reduction potentials.![E^0_{[Mg^{2+}/Mg]}= -2.37V](/tpl/images/0383/2083/24fc1.png)
![E^0_{[Al^{3+}/Al]}=-1.66V](/tpl/images/0383/2083/0867c.png)
![E^0=E^0_{[Mg^{2+}/Mg]}- E^0_{[Al^{3+}/Al]}](/tpl/images/0383/2083/0b323.png)
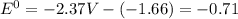

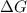 = gibbs free energy
= gibbs free energy