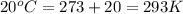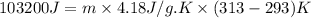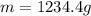Chemistry, 20.11.2019 20:31 bougiehairstudios
For many years drinking water has been cooled in hot climates by evaporating it from the surface of canvas bags or porous clay pots. how many grams of water can be cooled from 40 ∘c to 20 ∘c by the evaporation of 43 g of water? (the heat of vaporization of water in this temperature range is 2.4 kj/g. the specific heat of water is 4.18 j/g⋅k.)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 20:30
Which of the following true? a_volcanoes and earthquakes often near the plate boundaries. b_volcanoes occur whereve there are tall mountains. c_earthquakes cause volcanoes in the same location to erupt violently d_volcanoes and earthquakes occur only where plates are colliding with each other
Answers: 2

Chemistry, 22.06.2019 06:30
Predict whether the changes in enthalpy, entropy, and free energy will be positive or negative for the boiling of water, and explain your predictions. how does temperature affect the spontaneity of this process?
Answers: 1


Chemistry, 22.06.2019 08:30
Joan writes four numbers on the board in standard form, and then she writes their scientific notation
Answers: 1
You know the right answer?
For many years drinking water has been cooled in hot climates by evaporating it from the surface of...
Questions


Mathematics, 28.03.2021 05:30


Chemistry, 28.03.2021 05:30


English, 28.03.2021 05:30







Mathematics, 28.03.2021 05:30


Mathematics, 28.03.2021 05:30





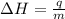
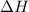 = enthalpy change or heat of vaporization = 2.4 kJ/g = 2400 J/g
= enthalpy change or heat of vaporization = 2.4 kJ/g = 2400 J/g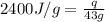
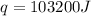
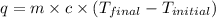
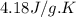
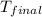 = final temperature =
= final temperature = 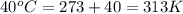
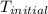 = initial temperature =
= initial temperature =