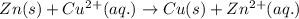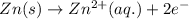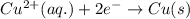Chemistry, 20.11.2019 17:31 fowers8376
Which species functions as the oxidizing agent in the following reduction-oxidation reaction:
zn(s) + cu^2+(aq) > cu(s) + zn^2+(aq)
a) zn^2+(aq)
b) zn(s)
c) cu^2+
d) cu(s)

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 23:00
What is the molecular formula for a compound that is 46.16% carbon, 5.16% hydrogen, and 48.68% fluorine? the molar mass of the compound is 156.12 g/mol
Answers: 2

Chemistry, 22.06.2019 00:00
What stress will shift the following equilibrium system to the left? n2(g) + 3h2(g) ⇌ 2nh3(g) adding more n2(g) adding more nh3(g) increasing the pressure of the system reducing the volume of the container
Answers: 1

Chemistry, 22.06.2019 07:10
Provide a stepwise curved arrow mechanism that fully explains the outcome of the reaction shown below. oh нао* heat он
Answers: 2

You know the right answer?
Which species functions as the oxidizing agent in the following reduction-oxidation reaction:
Questions

Mathematics, 30.06.2019 10:30



Mathematics, 30.06.2019 10:30

Computers and Technology, 30.06.2019 10:30

English, 30.06.2019 10:30

Mathematics, 30.06.2019 10:30




English, 30.06.2019 10:30


Mathematics, 30.06.2019 10:30



Mathematics, 30.06.2019 10:30

English, 30.06.2019 10:30



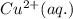 is the oxidizing agent for the given equation.
is the oxidizing agent for the given equation.