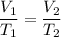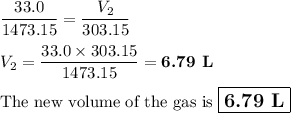Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 11:00
Iron (3) oxide will decompose in the presence of hydrogen gas and heater to produced iron and digydrogen monoxide white a balanced chemical equation
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1

Chemistry, 23.06.2019 00:50
50 points! need answer asap. what type of organic compound contains the following functional group? (2 points)
Answers: 3
You know the right answer?
calcium carbonate decomposes at 1200.0°c and forms carbon dioxide and calcium oxide. if 33.0 l of ca...
Questions


Social Studies, 05.05.2020 22:24

History, 05.05.2020 22:24


Mathematics, 05.05.2020 22:24

Social Studies, 05.05.2020 22:24




History, 05.05.2020 22:24



Chemistry, 05.05.2020 22:24

Mathematics, 05.05.2020 22:24




Chemistry, 05.05.2020 22:24