

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 05:50
In an exothermic reaction the bonding energy of the product is: less than the reactants same as the reactants greater than the reactants dependent upon the presence of a catalyst
Answers: 1

Chemistry, 23.06.2019 01:30
Will a solution form when the solvent and solute are both nonpolar? a. not likely b. never c. most likely
Answers: 1

Chemistry, 23.06.2019 04:20
The lewis diagrams for magnesium and fluorine are shown below. what is the correct chemical formula for magnesium fluoride? a. mgf b. mg2f c. mgf2 d. mg2f3
Answers: 1

Chemistry, 23.06.2019 14:30
Hydrogen has three isotopes 1h, 2h, and 3h. what is the difference between these three isotopes?
Answers: 2
You know the right answer?
Which of the following transitions represent the emission of a photon with the largest energy?
Questions

Computers and Technology, 10.12.2020 17:00






English, 10.12.2020 17:00

Advanced Placement (AP), 10.12.2020 17:00

Mathematics, 10.12.2020 17:00




Health, 10.12.2020 17:00



Mathematics, 10.12.2020 17:00



Mathematics, 10.12.2020 17:00

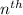 orbit is directly proportional to
orbit is directly proportional to 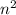 .Hence a transistion from one orbit to another orbit emits an energy proportional to the difference of their squares of the orbits. that is if an electron travels from orbit n1 to orbit n2 then it emits an energy corresponding to
.Hence a transistion from one orbit to another orbit emits an energy proportional to the difference of their squares of the orbits. that is if an electron travels from orbit n1 to orbit n2 then it emits an energy corresponding to  .So in the above question the highest energy emission occurs when an electron moves from n=6 to n=3.(Highest difference of energy levels).
.So in the above question the highest energy emission occurs when an electron moves from n=6 to n=3.(Highest difference of energy levels).

