
Chemistry, 20.11.2019 05:31 santiago172005
Classify the following bonds as ionic, polar covalent, or covalent, and give your reasons: (a) the cc bond in h3cch3, (b) the ki bond in ki, (c) the nb bond in h3nbcl3, (d) the cf bond in cf4.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 04:30
Use the periodic table to determine the electron configuration of dysprosium (dy) and americium (am) in noble-gas notation.
Answers: 1

Chemistry, 22.06.2019 06:40
Which statement is usually true about the relationship between activation energy and reaction rates? low activation energy barriers result in low rates. high activation energy barriers result in low rates. low activation energy barriers result in no reaction. high activation energy barriers result in no reaction.
Answers: 3

Chemistry, 22.06.2019 07:30
11. phosphorus-32 is radioactive and has a half life of 14 days. how much of a 124 mg sample of phosphorus-32 is present after 56 days? a) 7.75 mg b) 15.5 mg c) 31.0 mg d) 62.0 mg
Answers: 3

Chemistry, 22.06.2019 10:50
An atom of lithium-7 has an equal number of(1) electrons and neutrons(2) electrons and protons(3) positrons and neutrons(4) positrons and protons
Answers: 2
You know the right answer?
Classify the following bonds as ionic, polar covalent, or covalent, and give your reasons: (a) the...
Questions

Mathematics, 21.08.2019 03:00



Mathematics, 21.08.2019 03:00



English, 21.08.2019 03:00

Mathematics, 21.08.2019 03:00


Physics, 21.08.2019 03:00

Computers and Technology, 21.08.2019 03:00

Social Studies, 21.08.2019 03:00

Arts, 21.08.2019 03:00


History, 21.08.2019 03:00


English, 21.08.2019 03:00

History, 21.08.2019 03:00


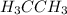 : covalent
: covalent 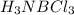 : polar covalent
: polar covalent 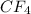 : polar covalent
: polar covalent 

