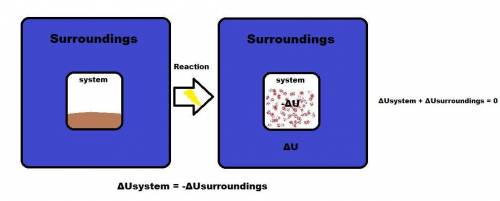
The internal energy can be increased by
(a) transferring heat from the surroundings to the sy...

Chemistry, 20.11.2019 02:31 fjjjjczar8890
The internal energy can be increased by
(a) transferring heat from the surroundings to the system.
(b) transferring heat from the system to the surroundings.
(c) doing work on the system.
a) a only.
b) b only.
c) c only.
d) a and c.
e) b and c.

Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 14:10
16. in a reaction that has reached equilibrium, a. the forward and reverse reactions are occurring at the same rate. b. the reactants and products are in equal concentrations. c. the forward reaction has gone further than the reverse reaction. d. there are equal numbers of atoms on both sides of the equation. e. a, b, and d are correct.
Answers: 2

Chemistry, 23.06.2019 00:00
In an exothermic reaction, energy may be released to the surroundings in the form of question 4 options: heat light thermal all of the above
Answers: 3

Chemistry, 23.06.2019 03:00
What volume does 1.70 ×10–3 mol of chlorine gas occupy if its temperature is 20.2 °c and its pressure is 795 mm hg?
Answers: 3

You know the right answer?
Questions

Arts, 14.11.2020 14:10





Mathematics, 14.11.2020 14:20



Mathematics, 14.11.2020 14:20


English, 14.11.2020 14:20

English, 14.11.2020 14:20

Social Studies, 14.11.2020 14:20



Mathematics, 14.11.2020 14:20

Mathematics, 14.11.2020 14:20

Spanish, 14.11.2020 14:20


Biology, 14.11.2020 14:30