
Chemistry, 20.11.2019 01:31 orlando19882000
You order a 16 oz glass of tea (where the mass of water is 474 grams) from a local restaurant. the tea is freshly brewed and has an initial temperature of 28.29 °c. you add ice to cool it. if the heat of fusion of ice is 6.020 kj/mol and each ice cube contains exactly 1 mol of water, how many ice cubes are necessary to cool the tea to 0.41 °c? the specific heat of the "tea" is 4.184 j/g*c.

Answers: 2


Another question on Chemistry

Chemistry, 21.06.2019 21:30
Sex cells from female reproductive organ? 1) mitosis 2) fertilization 3) zygote 4) eggs 5) meiosis 6) sperm
Answers: 2

Chemistry, 22.06.2019 15:40
Use the periodic table to complete this equation that represents nuclear fission processesun - ba c 3 n
Answers: 2

Chemistry, 22.06.2019 17:00
Which property of a rock remains unchanged by mechanical weathering? a. total surface area b. size and shape c. mineral composition d. sharpness
Answers: 1

Chemistry, 22.06.2019 21:50
What is a main difference between a mixture and a pure substance? a mixture is only a liquid, but a pure substance can be in any state.a mixture looks the same throughout, but a pure substance does not.1 a mixture can vary in composition, but a pure substance has a set composlo a mixture can be made up of a single compound, but a pure substance car
Answers: 2
You know the right answer?
You order a 16 oz glass of tea (where the mass of water is 474 grams) from a local restaurant. the t...
Questions

Mathematics, 02.04.2021 01:00

Geography, 02.04.2021 01:00





Mathematics, 02.04.2021 01:00





Geography, 02.04.2021 01:00

Mathematics, 02.04.2021 01:00

Mathematics, 02.04.2021 01:00

Mathematics, 02.04.2021 01:00

Arts, 02.04.2021 01:00

Geography, 02.04.2021 01:00


Mathematics, 02.04.2021 01:00

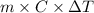
 = change in temperature =
= change in temperature = 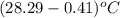 =
= 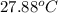
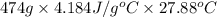
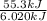
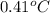 .
.

