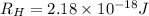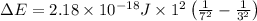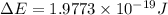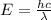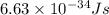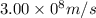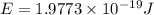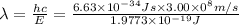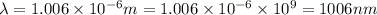Answers: 1


Another question on Chemistry

Chemistry, 22.06.2019 06:00
An alkaline battery produces electrical energy according to the following equation. zn(s) + 2 mno2(s) + h2o(l) zn(oh)2(s) + mn2o3(s) (a) determine the limiting reactant if 17.5 g zn and 31.0 g mno2 are used. (type your answer using the format ch4 for ch4.) (b) determine the mass of zn(oh)2 produced. _ g
Answers: 3

Chemistry, 22.06.2019 09:20
Sugar is dissolved in water. which is the solute? sugar neither both water
Answers: 1

Chemistry, 22.06.2019 13:30
Apush or pull that moves or changes and object when to objects touch
Answers: 2

Chemistry, 22.06.2019 14:10
Aconcentrated solution of ammonia is 14.8m and has a density of 0.899g/l. what is the concentration of ammonia in this solution in weight percent (%w/w)?
Answers: 1
You know the right answer?
Given that rh= 2.18 x 10⁻¹⁸j, 1 nm = 1 x 10⁻⁹m, h = 6.63 x 10⁻³⁴j·s, and c = 3.00 x 10⁸m/s:
c...
c...
Questions

Mathematics, 16.02.2021 21:50






History, 16.02.2021 21:50



Mathematics, 16.02.2021 21:50

Mathematics, 16.02.2021 21:50

Chemistry, 16.02.2021 21:50

Mathematics, 16.02.2021 21:50

Computers and Technology, 16.02.2021 21:50





Mathematics, 16.02.2021 21:50

Mathematics, 16.02.2021 21:50

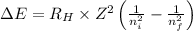
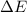 = Energy difference
= Energy difference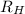 = Rydberg's Constant
= Rydberg's Constant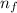 = Final energy level
= Final energy level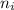 = Initial energy level
= Initial energy level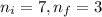 , Z = 1
, Z = 1