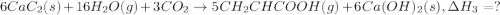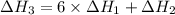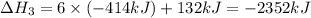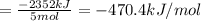Chemistry, 19.11.2019 23:31 sipstick9411
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of several useful plastics. in the first step, calcium carbide and water react to form acetylene and calcium hydroxide: (s) (g) (g) (s) in the second step, acetylene, carbon dioxide and water react to form acrylic acid: (g) (g) (g) (g) calculate the net change in enthalpy for the formation of one mole of acrylic acid from calcium carbide, water and carbon dioxide from these reactions. round your answer to the nearest .

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 15:40
What is the study of how matter and energy interact? a. biology b. physics c. planetary science d. chemistry
Answers: 1


Chemistry, 21.06.2019 22:30
Consider the following system at equilibrium. caco3(s) ca2+(aq) + co32–(aq) the addition of which compound will cause a shift in equilibrium because of a common ion effect? ccl4 co2 cuso4 na2co3
Answers: 3

Chemistry, 22.06.2019 20:40
What effect would average population growth have on land usage? a. urban use of land would rise to more than 30 percent of available land. b. industrial use of land would rise to more than 30 percent of available land. c. the percentage of available land used as cropland would stay the same. d. cropland would fall to about 10 percent of available land.
Answers: 1
You know the right answer?
There are two steps in the usual industrial preparation of acrylic acid, the immediate precursor of...
Questions


History, 29.01.2021 17:20

Biology, 29.01.2021 17:20


SAT, 29.01.2021 17:20

Social Studies, 29.01.2021 17:20

Mathematics, 29.01.2021 17:20





Medicine, 29.01.2021 17:20

Mathematics, 29.01.2021 17:20


Mathematics, 29.01.2021 17:20

Mathematics, 29.01.2021 17:20


Mathematics, 29.01.2021 17:20


Mathematics, 29.01.2021 17:20

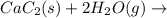
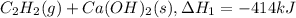 ..[1]
..[1]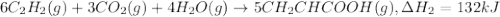 ..[2]
..[2]