
Chemistry, 19.11.2019 22:31 TheOneandOnly003
Given the solutes nacl and i2 and the solvents nh3(ℓ) and c7h16 (heptane), which is true?
nacl and i2 are both more soluble in nh3 than in c7h16.
nacl and i2 are both more soluble in c7h16 than in nh3.
nacl is more soluble in nh3; i2 is more soluble in c7h16.
nacl is more soluble in c7h16; i2 is more soluble in c7h16.
in order to determine relative solubilities in these cases one must know whether the solution reactions are exothermic or endothermic.

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 10:30
When the speed of the bottle is 2 m/s, the average maximum height of the beanbag is m.
Answers: 2



You know the right answer?
Given the solutes nacl and i2 and the solvents nh3(ℓ) and c7h16 (heptane), which is true?
Questions



Mathematics, 13.04.2020 01:16


Arts, 13.04.2020 01:29


Mathematics, 13.04.2020 01:29

English, 13.04.2020 01:29




Computers and Technology, 13.04.2020 01:29




Biology, 13.04.2020 01:29

Mathematics, 13.04.2020 01:29

Mathematics, 13.04.2020 01:29

Chemistry, 13.04.2020 01:29

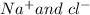 so it is soluble in polar compounds like ammonia (NH3),where as I2 is a non-polar solid so it is soluble in non-polar solvents like n-heptane(C7H16).
so it is soluble in polar compounds like ammonia (NH3),where as I2 is a non-polar solid so it is soluble in non-polar solvents like n-heptane(C7H16).

