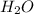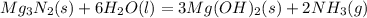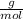Chemistry, 19.11.2019 18:31 nadiarose6345
Mg3n2(s)+6h2o(l)→3mg(oh)2(s)+2nh3(g ) when 36.0 g of h2o react, how many grams of nh3 are produced? when 36.0 g of h2o react, how many grams of nh3 are produced? 34.0 g 10.0 g 5.67 g 11.3 g 102 g

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 01:20
Match the acid base pairs by arranging the acid name with the conjugate base formula. hydrogen carbonate hydrogen phosphate carbonic acid read water sulfuric acid phosphoric acid a. co32- b. hso4- c. hco3- d. po43- e. h2po4- f. oh-
Answers: 1


Chemistry, 22.06.2019 03:30
At a temperature of 393 k, the temperature of a sample of nitrogen is 1.07 atm what will the pressure be at a temperature of 478 k
Answers: 1

You know the right answer?
Mg3n2(s)+6h2o(l)→3mg(oh)2(s)+2nh3(g ) when 36.0 g of h2o react, how many grams of nh3 are produced?...
Questions


Mathematics, 08.11.2019 01:31

English, 08.11.2019 01:31




Biology, 08.11.2019 01:31

History, 08.11.2019 01:31



History, 08.11.2019 01:31




History, 08.11.2019 01:31



Biology, 08.11.2019 01:31

Mathematics, 08.11.2019 01:31

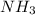 are produced from 36.0 g of
are produced from 36.0 g of