
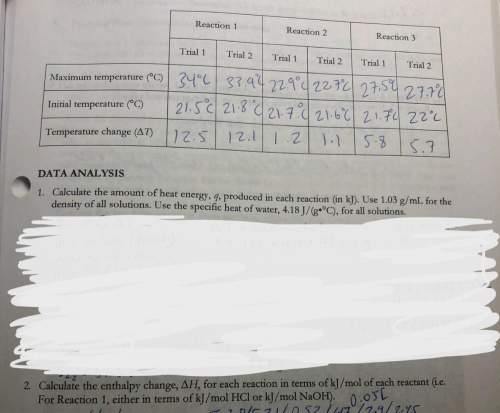
3. use your answers from 2 above and hess’s lawton determine the experimental molar enthalpy for reaction three.
4. use hess’s law, and the accepted values of change of h in the pre-lab exercise to calculate the change in h for reaction 3. how does the accepted value compare to your experimental value?

Answers: 2


Another question on Chemistry

Chemistry, 22.06.2019 05:30
The table describes how some substances were formed substance 19 description formed by boiling pure water formed by combining three hydrogen atoms to every nitrogen atom formed by adding 5 g of sugar to 1 l of water formed by compressing carbon under high pressure based on the given descriptions, which substance is most likely a mixture?
Answers: 1

Chemistry, 22.06.2019 07:00
The variability in marine salinity between habitats does not impact the fish living there. select the best answer from the choices provided t f
Answers: 1

Chemistry, 22.06.2019 15:30
Draw the lewis dot structure for each of the following polyatomic ions
Answers: 1

Chemistry, 23.06.2019 01:00
If i had 2 m naoh solution, what does the 2 m stand for? 2 molar, but 2 of a solute in 1
Answers: 1
You know the right answer?
3. use your answers from 2 above and hess’s lawton determine the experimental molar enthalpy for rea...
Questions

Mathematics, 12.11.2020 07:10


Biology, 12.11.2020 07:10

Mathematics, 12.11.2020 07:10


Physics, 12.11.2020 07:20

Mathematics, 12.11.2020 07:20




Chemistry, 12.11.2020 07:20

Biology, 12.11.2020 07:20

Mathematics, 12.11.2020 07:20


Physics, 12.11.2020 07:20


Mathematics, 12.11.2020 07:20


Mathematics, 12.11.2020 07:20

Mathematics, 12.11.2020 07:20



