Chemistry, 19.11.2019 02:31 kaylallangari2145
Hydrogen iodide, hi, is formed in an equilibrium reaction when gaseous hydrogen and iodine gas are heated together. if 20.0 g of hydrogen and 20.0 g of iodine are heated, forming 10.0 g of hydrogen iodide, what mass of hydrogen remains unreacted? a. 10.0 g hydrogen remains b. 10.9 g hydrogen remains c. 15.0 g hydrogen remains d. 19.9 g hydrogen remains.

Answers: 3


Another question on Chemistry

Chemistry, 21.06.2019 13:30
Which is the most likely way an automotive engineer would use chemistry
Answers: 1


Chemistry, 22.06.2019 10:50
A100 kmol/h stream that is 97 mole% carbon tetrachloride (ccl4) and 3% carbon disulfide (cs2) is to be recovered from the bottom of a distillation column. the feed to the column is 16 mole% cs2 and 84% ccl4, and 2% of the ccl4 entering the column is contained in the overhead stream leaving the top of the column. calculate the mass and mole fractions of ccl4 in the overhead stream, and determine the molar flow rates of ccl4 and cs2 in the overhead and feed streams. 12. mw_ccla- 153.82; mw_cs2-76.14.
Answers: 3

Chemistry, 22.06.2019 19:00
What is the compound name for the formula [ru(en)2cl2]2+ and [co(en)cl2br]-
Answers: 1
You know the right answer?
Hydrogen iodide, hi, is formed in an equilibrium reaction when gaseous hydrogen and iodine gas are h...
Questions





Mathematics, 22.04.2020 04:01


Mathematics, 22.04.2020 04:01




Mathematics, 22.04.2020 04:01




History, 22.04.2020 04:01





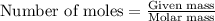
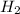
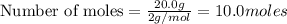
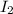
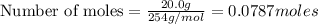
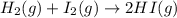
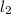 require=
require=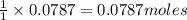 of
of