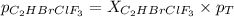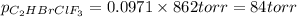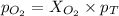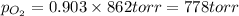Chemistry, 19.11.2019 02:31 marlenemedina247
Amixture of 15.0 g of the anesthetic halothane (c2hbrclf3 197.4 g/mol) and 22.6 g of oxygen gas has a total pressure of 862 torr. what are the partial pressures of each gas? a. phalothane = 778 torr, po2 = 84 torr b. phalothane = 162 torr, po2 = 700 torr c. phalothane = 84 torr, po2 = 778 torr d. phalothane = 155 torr, po2 = 707 torr e. phalothane = 707 torr, po2 = 155 torr.

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 12:00
Hey guys so i need to know what is _nh3+> nh4oh ~chemistry~
Answers: 1

Chemistry, 22.06.2019 14:30
Select all of the statements which are true. electrons are located in shells or orbits around the atom. electrons orbit slowly around the atom. electrons travel in one flat path around the nucleus of an atom. the valence of an atom is determined by the number of electrons in the atom's outermost shell.
Answers: 1

Chemistry, 23.06.2019 01:30
List and describe the neurological effects of the vocs and other air pollutants,as described by dr.theo colborn
Answers: 2

Chemistry, 23.06.2019 02:40
Calculate the standard enthalpy of formation of liquid methanol, ch3oh(l), using the following information: c(graphite) + o2 latex: \longrightarrow ⟶ co2(g) latex: \delta δ h° = –393.5 kj/mol h2(g) + o2 latex: \longrightarrow ⟶ h2o(l) latex: \delta δ h° = –285.8 kj/mol ch3oh(l) + o2(g) latex: \longrightarrow ⟶ co2(g) + 2h2o(l) latex: \delta δ h° = –726.4 kj/mol
Answers: 3
You know the right answer?
Amixture of 15.0 g of the anesthetic halothane (c2hbrclf3 197.4 g/mol) and 22.6 g of oxygen gas has...
Questions











History, 30.10.2020 17:10

Mathematics, 30.10.2020 17:10








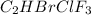 and
and 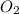 are, 84 torr and 778 torr respectively.
are, 84 torr and 778 torr respectively.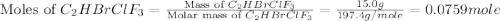
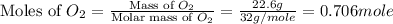
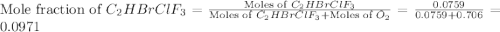
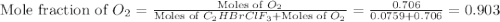
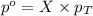
 = partial pressure of gas
= partial pressure of gas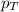 = total pressure of gas
= total pressure of gas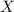 = mole fraction of gas
= mole fraction of gas