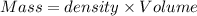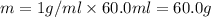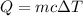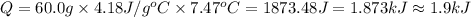Chemistry, 19.11.2019 02:31 Miloflippin9766
A50.0 ml of 0.88 m h2o2 and 10.0 ml of 0.50 m fe(no3)3 were combined and a temperature change of 7.47 was observed. the specific heat of water is 4.18 j/(g * ∘c) calculate the heat of reaction (in kilojoules). record your answer with the proper significant figures and include the correct sign if needed. assume the density and specific heat of the solution are the same as that of water. kj

Answers: 3


Another question on Chemistry

Chemistry, 22.06.2019 13:00
Is 9 correct? and can someone me with 10? it’s due tomorrow, you
Answers: 1


Chemistry, 23.06.2019 03:00
Which of the following is a chemical property of water at 4 c
Answers: 2

Chemistry, 23.06.2019 04:40
Equal numbers of moles of he(g), ar(g), and ne(g) are placed in a glass vessel at room temperature. if the vessel has a pinhole-sized leak, which of the following will be true regarding the relative values of the partial pressures of the gases remaining in the vessel after some of the gas mixture has effused?
Answers: 1
You know the right answer?
A50.0 ml of 0.88 m h2o2 and 10.0 ml of 0.50 m fe(no3)3 were combined and a temperature change of 7.4...
Questions

History, 05.11.2019 22:31






Mathematics, 05.11.2019 22:31





English, 05.11.2019 22:31


Mathematics, 05.11.2019 22:31


Mathematics, 05.11.2019 22:31