
Chemistry, 19.11.2019 02:31 YatesDevon3371
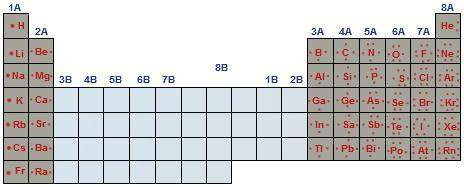
Describe the pattern in valence electrons on the periodic table.
a. valence electrons increase as you go from left to right across the table, and from top down to bottom of the table.
b. valence electrons increase as you go from right to left across the table and from top down to bottom of the table.
c. valence electrons increase as you go from left to right across the table, but are generally the same in each group.
d. valence electrons increase as you go from right to left across the table, but are generally the same in each group.

Answers: 2


Another question on Chemistry



Chemistry, 22.06.2019 20:00
Many free radicals combine to form molecules that do not contain any unpaired electrons. the driving force for the radical–radical combination reaction is the formation of a new electron‑pair bond. consider the chemical equation. n(g)+no(g)⟶nno(g) n(g)+no(g)⟶nno(g) write lewis formulas for the reactant and product species in the chemical equation. include nonbonding electrons. n(g)n(g) select draw rings more erase select draw rings more erase select draw rings more erase n no(g)
Answers: 1

Chemistry, 23.06.2019 01:00
Chromium(iii) sulfate is a transition metal compound containing the metal chromium and the polyatomic ion sulfate. the oxidation state of chromium in this compound is , and the chemical formula of the compound is ( ) . reset next
Answers: 3
You know the right answer?
Describe the pattern in valence electrons on the periodic table.
a. valence electrons in...
a. valence electrons in...
Questions


Chemistry, 10.04.2020 05:17

Social Studies, 10.04.2020 05:18

Mathematics, 10.04.2020 05:18

History, 10.04.2020 05:18

Chemistry, 10.04.2020 05:18


Physics, 10.04.2020 05:18

History, 10.04.2020 05:18


Mathematics, 10.04.2020 05:18


Biology, 10.04.2020 05:18

Mathematics, 10.04.2020 05:18

English, 10.04.2020 05:18



History, 10.04.2020 05:18




